Camera & Photo


 http://advancedmathematicalresearch.blogspot.in/
http://advancedmathematicalresearch.blogspot.in/ http://advancedmathematicalresearch.blogspot.in/
http://advancedmathematicalresearch.blogspot.in/ http://advancedmathematicalresearch.blogspot.in/
http://advancedmathematicalresearch.blogspot.in/ http://advancedmathematicalresearch.blogspot.in/
http://advancedmathematicalresearch.blogspot.in/ http://advancedmathematicalresearch.blogspot.in/
http://advancedmathematicalresearch.blogspot.in/ http://advancedmathematicalresearch.blogspot.in/
http://advancedmathematicalresearch.blogspot.in/ Name Your Link
Name Your Link Name Your Link
Name Your Link Name Your Link
Name Your Link Name Your Link
Name Your Link Name Your Link
Name Your Link Name Your Link
Name Your Link Name Your Link
Name Your Link
Today i will continue from Redox reaction in Bio chemistry.
We will see that the Spontaneous Redox Reaction whose Gibb's Free Energy is negative or equal to zero.
 favoured reaction (Spontaneous) reaction
favoured reaction (Spontaneous) reaction
Which is defined in terms of redox reduction potential as

http://advancedmathematicalresearch.blogspot.in/We will see that the Spontaneous Redox Reaction whose Gibb's Free Energy is negative or equal to zero.
then
Which is defined in terms of redox reduction potential as
Useful identities
<0, for Spontaneous Reaction
,Where K=Equilibrium constant
Qr=Reaction Quotient in Chemical reaction
- Qr=K ,when Change in Gibb;s free energy is equal to zero
<0, For Spontaneous Redox Reaction
-
- These are some Equations helpful to understand the relation among Gibb's free Energy change,Equilibrium constant and Spontaneity of Redox reaction in Photosynthesis light reaction.
- This Gibb's Energy change ie
<0 , during redox reaction which is photosynthesis light reaction gets stores in ATP Phosphodiester bond as dealt in my previous lecture and blog. Whose figure i can show now
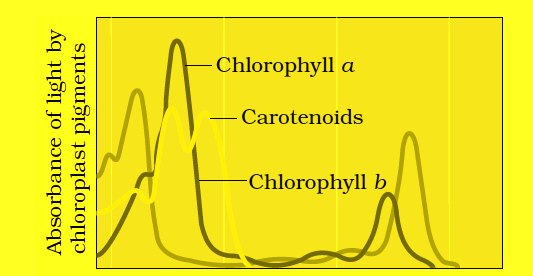
- Okey come to our topic of Photosynthesis light reaction

- Conclusion
- It is the negative of Gibb's free energy change in Redox reaction which gets stored in the form of ATP during Photo-phosphorylation reaction in Photosynthesis Light Reaction.
- I will deal with many correlated topics in Engineering, Bio chemistry ,Mathematics,Physics and Chemistry in the coming Session which will have a common origin.






















No comments:
Post a Comment