http://advancedmathematicalresearch.blogspot.in/

Today's topic is
1.Energy and Metabolism
2.Free-Energy Changes in Biological Systems.
3.Thermodynamics
I. Free energy of formation
II. Effect of temperature on spontaneity
III. Thermodynamics in biological systems.
A. ATP-coupled reactions
B. Hydrogen bonding
The laws of thermodynamics describe how energy changes. The Flow of Energy in Living Things. Potential energy is present in the valance electrons of atoms, and so can be transferred from one molecule to another.
The Laws of Thermodynamics. Energy is never lost but as it is transferred, more and more of it dissipates as heat, a disordered form of energy. Free Energy. In a chemical reaction, the energy released or supplied is the difference in bond energies between reactants and products, corrected for disorder.
Activation Energy. To start a chemical reaction, an input of energy is required to destabilize existing chemical bonds
Enzymes are biological catalysts and lowers the activation energy required by any reactant to convert to product by passing through the transition state called Activated state. Thus it helps lowering activation energy of any reactant and thus increase the rate constant as supported by following figure and formula.
Thus the activation energy has been lowered by using Bio catalyst called catalyst .Other than enzyme the rate constant depends upon temperature It has been found that for a chemical reaction with rise in temperature by 10°, the rate constant is nearly doubled.The temperature dependence of the rate of a chemical reaction can
be accurately explained by Arrhenius equation It was first proposed by Dutch chemist, J.H. van’t Hoff but Swedish chemist, Arrhenius provided its physical justification and interpretation.
be accurately explained by Arrhenius equation It was first proposed by Dutch chemist, J.H. van’t Hoff but Swedish chemist, Arrhenius provided its physical justification and interpretation.
k = A e -Ea /RT (Arrhenius formula)
where A is the Arrhenius factor or the frequency factor. It is also called pre-exponential factor. It is a constant specific to a particular reaction. R is gas constant and Ea is activation energy measured in joules/mole (J mol –1).
It can be understood clearly using the following simple reaction
H2(g) + I2( g) → 2HI(g).
According to Arrhenius, this reaction can take place only
when a molecule of hydrogen and a molecule of iodine collide
to form an unstable intermediate.The energy required to form this
intermediate, called activated complex (C), is known as activation
energy (Ea).
when a molecule of hydrogen and a molecule of iodine collide
to form an unstable intermediate.The energy required to form this
intermediate, called activated complex (C), is known as activation
energy (Ea).
Taking natural logarithm of both sides of Arrhenius equation we get
ln k = – Ea/ RT + ln A
Applying this equation at two different temperatures T1 and T2 such that T2>T1.
At T1
ln k1 = –E/RT1 + ln A
At T2
ln k2 = –Ea/RT2 + ln A
(since A is constant for a given reaction) k1 and k2 are the values of rate constants at temperatures T1 and T2 respectively.
ln k2 – ln k1 =Ea/RT1 –Ea/ RT2.
ln(K2/K1)=-Ea/R[1/T2-1/T1].
Plot is as following.
To deal with energetics in bio-chemistry we have to deal with thermodynamics and thermodynamics associated with Redox reaction.
ENERGIES AND ENTHALPIES OF CHEMICAL REACTIONS
BOND ENERGY/ BOND ENTHALPY
change in H, bond dissociation Enthalpy , is the change in heat accompanying the dissociation of a bond (measured at constant pressure P).
Enthalpy is a “STATE” FUNCTION, which means H is independent of path.
Hess's Law: If two or more chemical equations are added to give another chemical equation, corresponding Enthalpies or Gibb's free energy or Entropy must be added ie Hess's law is true for Enthalpy,Gibb's free energy and Entropy of any reaction.
Equavalently it says the following
And this is the decomposition theory of energetics.
Another example is
Lets come to enzymes and its energetics and see how it lowers the activation energy by binding the substrate to the active site.All these concepts are associated with energetics i will come to energetics in terms of oxidation and reduction reaction in Bio molecules.
You will be amazed to know that the energetics also coming into picture in all biological reaction whether it would be the process of reduction of NAD+ to NADH+P or any biological reaction Enzymes come into picture to lower the Ea=Activation Energy which we have already discussed.
Let's illustrate it by picture.
An oxidation-reduction reaction. Cells use a chemical called NAD+ to carry out oxidation-reduction reactions. Energetic electrons are
often paired with a proton as a hydrogen atom. Molecules that gain energetic electrons are said to be reduced, while ones that lose
energetic electrons are said to be oxidized. NAD+ oxidizes energy-rich molecules by acquiring their hydrogens (in the figure, this proceeds
1→2→3) and then reduces other molecules by giving the hydrogens to them (in the figure, this proceeds 3→2→1).
Diagrammatically this can be represented as
Where mechanism of this oxidation and reduction reaction can be explained by this following equation
1)Arrhenius equation
The Arrhenius equation gives the quantitative basis of the relationship between the activation energy and the rate at which a reaction proceeds. From the Arrhenius equation, the activation energy can be found through the relation
2)Gibb's free energy Equation or entropy Equation
total Entropt change>0
Entropy change(System)+Entropy Change(Surrounding)>0.
And if you solve it
To derive the Gibbs free energy equation for an isolated system, let Stot be the total entropy of the isolated system, that is, a system that cannot exchange heat or mass with its surroundings. According to the second law of thermodynamics:
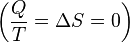 process.
process.Now consider a system having internal entropy Sint. Such a system is thermally connected to its surroundings, which have entropy Sext. The entropy form of the second law applies only to the closed system formed by both the system and its surroundings. Therefore a process is possible only if
 .
.
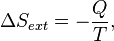 corresponds to the entropy change of the surroundings.
corresponds to the entropy change of the surroundings.We now have:
 for any chemical reaction to be spontaneous
for any chemical reaction to be spontaneous
Notice that it is not defined in terms of any external state functions, such as ΔSext or ΔStot. Then the second law, which also tells us about the spontaneity of the reaction, becomes:
http://advancedmathematicalresearch.blogspot.in/
 favoured reaction (Spontaneous)
favoured reaction (Spontaneous) Neither the forward nor the reverse reaction prevails (Equilibrium)
Neither the forward nor the reverse reaction prevails (Equilibrium) disfavoured reaction (Nonspontaneous)
disfavoured reaction (Nonspontaneous)

Enzymes typically catalyze only one or a few similar
chemical reactions because they are specific in their
choice of substrates. This specificity is due to the active
site of the enzyme, which is shaped so that only a
certain substrate molecule will fit into it.
The catalytic cycle of an enzyme. Enzymes increase the speed with which chemical reactions occur, but they are not altered
themselves as they do so. In the reaction illustrated here, the enzyme sucrase is splitting the sugar sucrose (present in most candy) into
two simpler sugars: glucose and fructose. (1) First, the sucrose substrate binds to the active site of the enzyme, fitting into a depression
in the enzyme surface. (2) The binding of sucrose to the active site forms an enzyme-substrate complex and induces the sucrase
molecule to alter its shape, fitting more tightly around the sucrose. (3) Amino acid residues in the active site, now in close proximity to
the bond between the glucose and fructose components of sucrose, break the bond. (4) The enzyme releases the resulting glucose and
fructose fragments, the products of the reaction, and is then ready to bind another molecule of sucrose and run through the catalytic
cycle once again. This cycle is often summarized by the equation: E + S ↔ [ES] ↔ E + P, where E = enzyme, S = substrate, ES =
enzyme-substrate complex, and P = products.
How Enzymes Work
Most enzymes are globular proteins with one or more pockets or clefts on their surface called active sites as shown above figure Substrates bind to the enzyme at these active sites, forming an enzyme-substrate complex. For catalysis to occur within the complex, a substrate molecule must fit precisely into an active site. When that happens, amino acid side groups of the enzyme end up in close proximity to certain bonds of the substrate. These side groups interact chemically with the substrate, usually stressing or distorting a particular bond and consequently lowering the activation energy needed to break the bond. The substrate, now a
product, then dissociates from the enzyme. Proteins are not rigid. The binding of a substrate induces the enzyme to adjust its shape slightly, leading to a better induced fit between enzyme and substrate refer figure above .This interaction may also facilitate the binding of othersubstrates; in such cases, the substrate itself “activates” the enzyme to receive other substrates.
So for most of the Biological and chemical reaction Gibb's energy change is paramount in driving a reaction forward or backward because Gibb's Energy change considers both Enthalpy change del(H) and Entropy change del(S) thus it is the complete relation for predicting the spontaneity of any chemical or biological process.The motivation of any event or process in Biological system is governed by to make del(G)<0.
THERMODYNAMICS IN BIOLOGICAL SYSTEMS A) ATP-COUPLED REACTIONS
Many biological reactions are non-spontaneous, meaning they require energy to proceed in the forward direction.
The hydrolysis of ATP (ATP → ADP), a spontaneous process, can be Coupled to a non-spontaneous reaction to drive the reaction forward.
The resulting ΔGº of the coupled reaction is the sum of the individual ΔGº values. First, let’s calculate the ΔGº for ATP hydrolysis at 310 K (body temperature).
ΔH° = -24 kJ/mol, ΔS° = +22 J/K•mol
Example of an ATP-coupled reaction: the conversion of glucose to glucose-6-P.
Adding a phosphate (P) group to glucose gives the glucose a negative charge, which prevents the glucose molecule from diffusing back out of the cell through the“greasy” cell membrane.
An enzyme couples the glucose-to-glucose-6-P reaction to ATP hydrolysis. The net change in free energy =
If ATP hydrolysis is spontaneous, why is it not occurring unregulated in the cell?
KINETICS! A reaction can be thermodynamically spontaneous, but kinetically very very slow.
HYDROGEN BONDING
A hydrogen bond is an electrostatic interaction between a hydrogen atom in a polar bond (typically a H-F, H-O or H-N bond) and a “hydrogen-bond donor”, a strongly electronegative atom.
The H-bond donor (Y) atom must be small, highly electronegative atom with a a Lone pair of electrons available for bonding.
For example, hydrogen bonds form between water molecules:
H-bonding can be intermolecular (as in the water molecules above) or intramolecular.Intramolecular H-bonds in proteins are required for a protein’s 3-dimensional shape.
In next blog i will come with some unique examples of Energetics in Bio-molecules and their process of synthesis inside the cell and you will come to know that it is the negative Gibb's energy of a Redox reaction del(G)= nFE(Reduction) which is stored during Photosynthesis in the plant in the form of Glucose ,ATP ,NADPH etc..
To see my lectures on the related topic click and Subscribe to my channel at the following address.
https://www.youtube.com/watch?v=n6fDwt8qEEI
https://www.youtube.com/watch?v=YvDP5bsasO0
https://www.youtube.com/watch?v=f4cqAGSFopw
https://www.youtube.com/watch?v=j-HfjXvrdNc
https://www.youtube.com/watch?v=nXry4GFtCwo
https://www.youtube.com/watch?v=Tz48_83IaYI
https://www.youtube.com/watch?v=pF4SpXpeVLE
https://www.youtube.com/watch?v=fe0OsoaXkfM
https://www.youtube.com/watch?v=dL5lBxZ68WE























No comments:
Post a Comment