First let's start with spacial orientation of a carbon compound.
Three-Dimensional Representation of Organic Molecules.
Hydrogen Attached by Dashed wedge is telling that H is having Spacial orientation and is pointing inside the paper and the H represented by solid wedge is telling that H is having a spacial orientation and pointing towards you ie above the paper.
If we try representing this on 2d Euclidean plane then the H that is pointing Above the paper is represented on the Right side of the vertical line formed by carbon chain and the H that is pointing inside the page is represented to the left of the vertical line formed by carbon chain and this Spacial orientation of substituent group is responsible for two types of sterioisomers. 1.Enantiomers: 2.Diastereomers:
Enantiomers are two stereoisomers that are related to each other by a reflection:They are mirror image of each other, which are non-superimposable. Human hands are a macroscopic analog of stereoisomerism. Every stereogenic center in one has the opposite configuration in the other. Two compounds that are enantiomers of each other have the same physical properties, except for the direction in which they rotate polarized light and how they interact with different optical isomers of other compounds. As a result, different enantiomers of a compound may have substantially different biological effects. Pure enantiomers also exhibit the phenomenon of optical activity and can be separated only with the use of a chiral agent. In nature, only one enantiomer of most chiral biological compounds, such as amino acids (except glycine, which is achiral), is present.
Diastereomers are stereoisomers not related through a reflection operation. They are not mirror images of each other. These include meso compounds, cis–trans (E-Z) isomers, and non-enantiomeric optical isomers. Diastereomers seldom have the same physical properties. In the example shown below, the meso form of tartaric acid forms a diastereomeric pair with both levo and dextro tartaric acids, which form an enantiomeric pair.
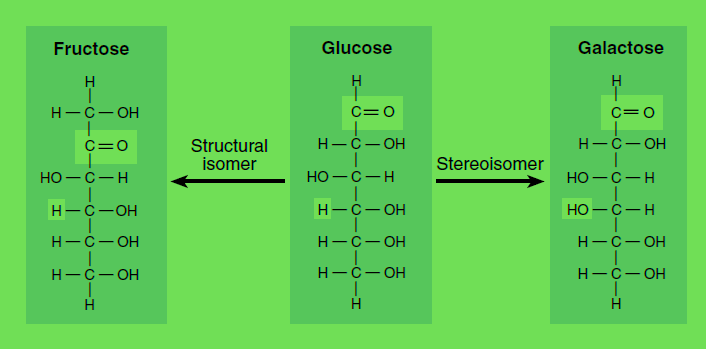
As an another example we can consider Glucose and Fructose as optically Active this as Sterio isomers. Thus the figure for this is as following:
Isomers and stereoisomers. Glucose, fructose, and galactose are isomers with the empirical formula C6H12O6. A structural isomer of glucose, such as fructose, has identical chemical groups bonded to different carbon atoms, while a stereoisomer of glucose, such as galactose, has identical chemical groups bonded to the same carbon atoms but in different orientation
Geometrical isomerism:
Stereoisomerism about double bonds arises because rotation about the double bond is restricted, keeping the substituents fixed relative to each other. If the two substituents on at least one end of a double bond are the same, then there is no stereoisomer and the double bond is not a stereocenter, e.g. propene, CH3CH=CH2 where the two substituents at one end are both H.
The isomer of the type (a), in which two identical atoms or groups lie on the same side of the double bond is called cis isomer and the other isomer of the type (b), in which identical atoms or groups lie on the opposite sides of the double bond is called trans isomer . Thus cis and trans isomers have the same structure but have different configuration (arrangement of atoms or groups in space). Due to different arrangement of atoms or groups in space, these isomers differ in their properties like melting point, boiling point, dipole moment, solubility etc.
Geometrical or cis-trans isomers of but-2-ene are as following
Streric effect ,Dipole effect etc is cancelled out in Trans form according to vector law of Subtraction since the two methyl group are vertically opposite to each other. Noe you can appreciate why?
Cis form of alkene is found to be more polar than the trans form. For example, dipole moment of cis-but-2-ene is 0.33 Debye, whereas, dipole moment of the trans form is almost zero or it can be said that trans-but-2-ene is non-polar. This can be understood by drawing geometries of the two forms as given below from which it is clear that in the trans-but-2-ene, the two methyl groups are in opposite directions,Threfore, dipole moments of C-CH3 bonds cancel, thus making the trans form non-polar.
Applying vector law of addition is equivalent ti vector subtraction of two dipole effect created by vertically opposite placed methyl group Diagrammatically this vector substraction can be shown as
Conformers:Conformational isomerism is a form of isomerism that describes the phenomenon of molecules with the same structural formula but with different shapes due to rotations about one or more bonds. Different conformations can have different energies, can usually interconvert, and are very rarely isolatable.
The Eclipsed is least stable with Gibb's energy change as positive and Staggered as the most stable as with change in Gibb's energy negative thus high equilibrium constant.
The Staggered form obtained by 180 degree of rotation of eclipsed form is the most stable because the two methyl/Substituent group are vertically opposite to each other thus providing least steric repulsion to each other making Gibb's free energy change negative and you can appreciate this change as the difference in the Enthalpy change in Eclipsed and staggered is 12KJ/mole.
This can well be represented by Figure as shown below.
Like us on facebook:https://www.facebook.com/pages/Advanced-Mathematical-Research-Group/456525267823638?ref=br_tf
https://www.youtube.com/watch?v=AqPpOgrdVhY
https://www.youtube.com/watch?v=QvkXccglyUc
https://www.youtube.com/watch?v=2KzXze22SOU
https://www.youtube.com/watch?v=2KzXze22SOU
https://www.youtube.com/watch?v=QNnhHRhXlYc
https://www.youtube.com/watch?v=nXry4GFtCwo
https://www.youtube.com/watch?v=n6fDwt8qEEI
https://t.co/8cyR1up9JQ Gravitational field is the distortion of Space Time 4D fabric by mass m making plane convex d pic.twitter.com/MWkPlGK5U0
— Abraham Malik (@brhmmlk93_malik) February 8, 2015